Tungsten carbide (WC) powder is a compound of tungsten and carbon, with the molecular formula WC and a molecular weight of 195.85. Typically, it appears as a gray or black powder with a metallic luster. This powder has hardness comparable to diamond and is an excellent conductor of electricity and heat. WC powder is insoluble in water, hydrochloric acid, and sulfuric acid but dissolves in a mixture of nitric and hydrofluoric acids.
|
Property |
Value |
Unit |
|
Chemical Formula |
WC |
- |
|
Molecular Weight |
195.86 |
g/mol |
|
Density |
15.6 |
g/cm³ |
|
Bulk Density |
2.5 – 6.5 |
g/cm³ |
|
Melting Point |
2,870 |
°C |
|
Boiling Point |
6,000 |
°C |
|
Mohs Hardness |
9 |
- |
|
Vickers Hardness |
2,200 – 2,800 |
HV |
|
Microhardness |
17 300 |
MPa |
|
Compressive Strength |
4,300 |
MPa |
|
Elastic Modulus |
530 – 700 |
GPa |
|
Thermal Conductivity |
84 – 100 |
W/m·K |
|
Thermal Expansion Coefficient |
4.3 – 5.9 |
µm/m·K |
|
Specific Heat (25°C) |
280 |
J/kg·K |
|
Electrical Resistivity |
2 x 10⁻⁷ |
Ω·m |
|
Particle Size |
Nano to Micron scale |
nm - µm |
|
Crystal Structure |
Hexagonal close-packed (HCP) |
- |
These properties make WC powder ideal for applications requiring high hardness, wear resistance, and heat tolerance, such as cutting tools, wear-resistant parts, thermal spraying, and 3D printing.
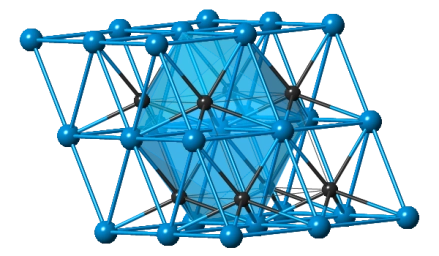
Fig 1. WC Powder Molecular Structure
Tungsten carbide (WC) powder usually has a hexagonal close-packed (HCP) structure, which gives it high density and hardness. In this structure, carbon atoms occupy interstitial sites between tungsten atoms, forming strong covalent bonds. This tight structure enhances the hardness and wear resistance of tungsten carbide.
Notably, the HCP structure exhibits directional properties, which can be leveraged in certain applications, such as optimizing cutting tool orientation for improved cutting efficiency.
In 1893, German scientists made use of tungsten trioxide and carbon heated together in an electric furnace to a high temperature to produce tungsten carbide, and tried to use its high melting point, high hardness and other characteristics to replace the diamond material. However, due to the brittleness of the material produced and easy to crack and other reasons, it has not been used in industrial applications. With gradual exploration, scientists have also researched a variety of methods to prepare tungsten carbide powder.
.jpg)
Fig 2. Tungsten Carbide Powder (WC Powder)
This method involves two steps. Ammonium tungstate or tungsten oxide is first reduced to produce tungsten powder. The tungsten powder is then mixed with carbon powder (usually graphite) and heated to 1400–2000°C in an inert atmosphere to produce tungsten carbide powder.
Reaction Equations:
(NH4)2WO4 + 3H2 → W + 2NH3 + 3H2O
W + C → WC
This process is relatively low cost, requires high temperature and inert gas protection, and is usually used for industrial mass production. However, the powder particles usually have irregular shapes and a rough surface.
This method reacts tungsten chlorides (e.g., WCl₆) and hydrocarbons (e.g., methane) in an inert atmosphere at high temperatures to produce tungsten carbide powder.
Reaction equation:
WCl₆ + CH₄ → WC + HCl
The result is a high-purity, ultra-fine tungsten carbide powder, ideal for high-performance and fine-grain applications, although production costs are high.
In this process, tungsten powder and carbon powder are milled in a high-energy ball mill. This mechanical energy during milling inside the ball mill induces an atomic reaction that produces tungsten carbide powder. With such a technique, ultra-fine powder is available at low temperature, but it requires a long grinding time; therefore, it is suitable for small-scale production.
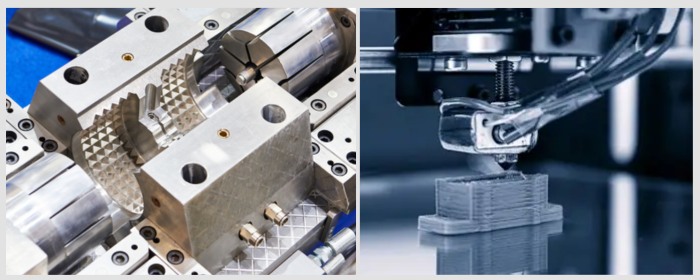
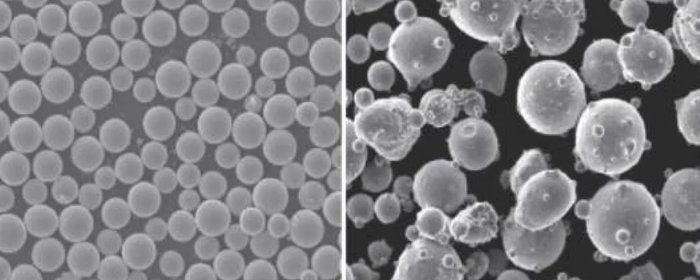
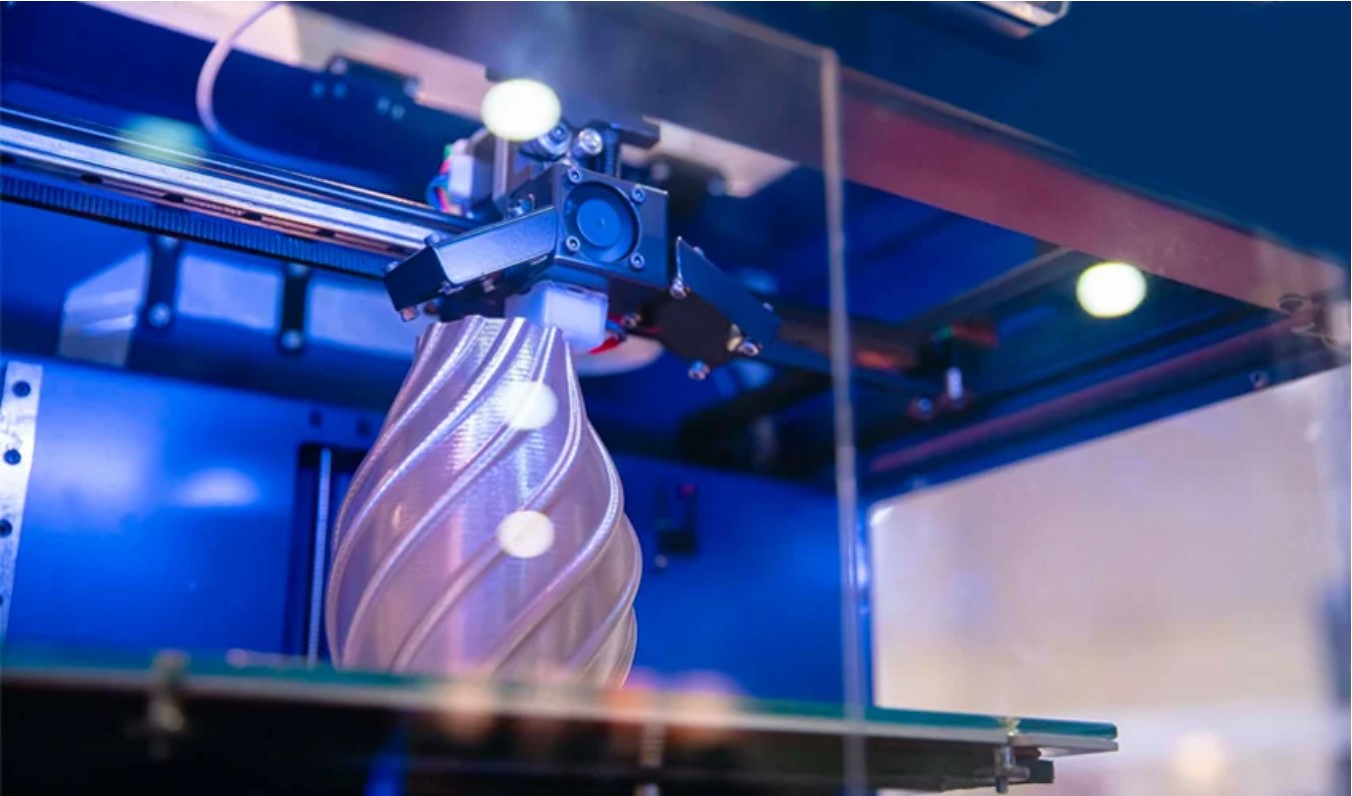
In this method, tungsten and carbon sources are exposed to high temperatures in a plasma stream to form tungsten carbide powder. It rapidly produces high-purity and spherical tungsten carbide powder, suitable for applications like 3D printing and thermal spraying, though it requires specialized equipment and is costlier.
As a key component in hard alloys, tungsten carbide powder plays an essential role in manufacturing cutting tools, molds, wear-resistant parts, and surface coatings.
Pure tungsten carbide is usually combined with metals such as titanium or cobalt to achieve toughness for use in specific applications. Additions such as titanium carbide or tantalum carbide are provided in the tungsten carbide cutting tools to improve their ability to resist fracturing.
Besides, different varieties of powders made by tungsten carbide have different performances and hence the difference in their applications. Table 2. shows the performance and application-related differences of the most important types of WC powders present in the market.
Table 2. Differences in properties and related uses of several major WC powders
|
Type |
Hardness |
Wear Resistance |
Toughness |
Shape |
Preparation Method |
Applications |
|
Cast WC Powder |
High |
High |
Moderate |
Irregular or spherical |
Molten casting and cooling |
Wear parts, impact-resistant components |
|
Fused WC Powder |
Very high |
Very high |
Low |
Irregular |
Electric arc melting and cooling |
High-wear environments, mining tools |
|
Sintered WC Powder |
High |
High |
Balanced |
Polycrystalline |
Powder metallurgy sintering |
Cutting tools, molds |
|
Nano WC Powder |
Extremely high |
Extremely high |
Low |
Nanoparticles |
Vapor deposition or mechanical alloying |
High-performance coatings, microelectronics |
|
Cemented WC Powder |
High |
High |
High |
Composite |
Sintering of WC with binders |
Cutting tools, stamping dies |
|
Ultra-Fine WC Powder |
High |
High |
Moderate |
Fine particles |
Fine grinding |
Precision abrasives, high-performance tools |
|
Spherical Tungsten Carbide Powder |
High |
High |
Moderate |
Spherical |
Gas atomization or plasma spraying |
3D printing, thermal spraying |
Note: Fusion casting cooling and electric arc furnace cooling are traditional preparation methods that involve melting tungsten and carbon at extremely high temperatures and cooling to form tungsten carbide particles. These methods focus on achieving high hardness and wear resistance, though the particle shape tends to be less fine and uniform compared to advanced methods like plasma synthesis.
Tungsten carbide powder, with its remarkable hardness, thermal stability, and wear resistance, is a crucial material in industries that demand high-performance components. From traditional methods like casting to advanced techniques like plasma synthesis, each production approach yields unique properties suited for specific applications. Whether for cutting tools or wear-resistant parts, tungsten carbide powder continues to be invaluable in extending the lifespan and functionality of industrial components.
Stanford Advanced Materials (SAM) provides high purity Tungsten Carbide Powder. If you are interested, please Get A Quote and learn more details.
Other related products