

As a critical functional metal material, copper powder is irreplaceable in various industrial fields. As the discipline of materials science advances, preparation technologies for ultrafine copper powder, with particle diameters ranging from 1 nm to 1 μm, have continued to lead the way, further expanding its application field in high-end production, electronics, energy, and other industries.
Copper is highly conductive electrically and thermally, and it's also very ductile, hence an industrial requirement. Copper is electrically second only to silver but much cheaper than silver powder, so it's a common option as a conductive filler or base of conductive pastes in the electronics industry. From the thermal conductivity point of view, the high thermal conductivity of copper as a material is suitable for heat dissipation applications, which are typically applied to thermal management systems of electronics. Copper powder is also featured by good plasticity and oxidation resistance, thus copper powder can be fabricated into products with various sizes and shapes through different fabrication paths.

Fig 1. Copper conductor
The traditional uses of copper powder include powder metallurgy, friction material, and chemical catalysts. For example, in powder metallurgy, copper powder can be pressed and sintered to produce high-strength mechanical parts. In friction material, copper powder is utilized to produce brake pads and clutch plates since it possesses excellent wear resistance and heat conductivity. With the development of nanotechnology, the applications of ultrafine copper powder have been extended even more, with high potential in new emerging fields such as high-precision electronic components, new energy materials, and 3D printing.
The preparative methods of ultrafine copper powder are diverse, predominantly including chemical reduction, electrochemical process, vapor-phase process, and mechanical ball milling. One of the most extensively used methods currently is chemical reduction, where copper ions in copper salt solution are reduced to copper powder with the aid of reducing agents, such as hydrazine hydrate or sodium borohydride, as shown in Figure 2. This process is easy to conduct and can yield nanoscale copper powder, but rapid reduction rates can cause agglomeration of particles, which influences the dispersibility of the final product.
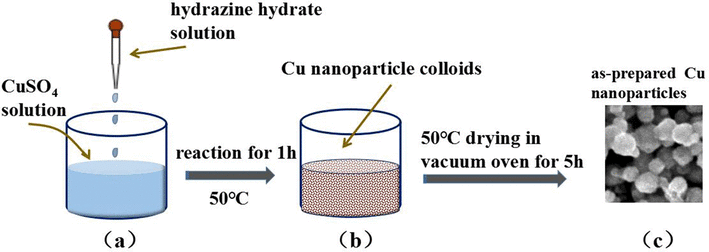
Fig 2. chemical reduction[1]
The electrochemical process entails the deposition of high-purity ultrafine copper powder on the cathode surface through the control of current density and electrolyte composition. It enjoys high purity and controllable morphology of copper powder, but low production efficiency, which can barely meet the demands of large-scale industrial applications.
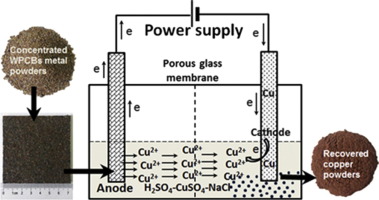
Fig 3. electrochemical process[2]
Vapor-phase methods are able to produce surface-clean, nano-sized copper powder with uniform size, but their equipment is costly, and they can be applied only to high-value-added products. Mechanical ball milling is possible and simple, but it is easy to introduce impurities during grinding and hard to achieve nanoscale powder.
Despite significant progress in the preparation technologies of ultrafine copper powder, there remain some crucial issues. For instance, nano-copper is extremely prone to oxidation and requires strict protection during the preparation process and storage. How to maintain product consistency and stability at large-scale production is another significant factor limiting industrial applications.
Ultrafine copper powder possesses broad application prospects in various emerging industries. In electronics, it is applied to manufacture high-conductive electronic pastes and printed circuit boards with properties comparable to silver paste but at lower cost, which makes it very competitive in the marketplace. The other important application lies in the new energy industry, in which copper powder is employed as a conductive additive in lithium-ion batteries and as a carrier of the catalyst in hydrogen fuel cells, significantly enhancing battery performance and service life.
The rapid development of 3D printing technology has opened up new application areas for ultrafine copper powder. Due to its excellent thermal conductivity and plasticity, copper powder has been the best material for 3D printing complex heat dissipation devices and electronic components. In aerospace applications, ultrafine copper powder is used to prepare lightweight, high-strength composite materials with performance demands under severe environments.
The medical industry has also begun to research the applications of ultrafine copper powder, such as the use of copper ions' antibacterial properties in antimicrobial materials and drug delivery systems. Ultrafine copper powder also has specific advantages in flexible electronics, smart wearable devices, environmental catalysts, and high-end chemical industries. As technology further evolves, these areas will become the primary sources of future growth for ultrafine copper powder.
Ultrafine copper powder possesses excellent properties and extensive application possibilities. Although there are still challenges in preparation technologies and industrialized applications, with the development of related technologies and the growing market demand, ultrafine copper powder will play an increasingly important part in future high-tech industries.
Stanford Advanced Materials (SAM) offers nano-sized and micron-sized spherical copper powder. Research or educational institutions may qualify for a 20% discount. For more information, please Get A Quote.
[1] Shang, Shengyan & Kunwar, Anil & Yao, Jinye & Wang, Yunpeng & Zhao, Ning & Ma, Haitao. (2018). All-round suppression of Cu6Sn5 growth in Sn/Cu joints by utilizing TiO2 nanoparticles. Journal of Materials Science Materials in Electronics. 29. 15966–15972. 10.1007/s10854-018-9682-z.
[2] Yingying Chu, Mengjun Chen, Shu Chen, Bin Wang, Micro-copper powders recovered from waste printed circuit boards by electrolysis,
Hydrometallurgy, Volume 156, 2015, Pages 152-157, ISSN 0304-386X, https://doi.org/10.1016/j.hydromet.2015.06.006.

United States
.png)



