

In modern industrial production, powder flowability is one of the key factors affecting processing efficiency and product quality. Whether in pharmaceutical tableting, ceramic sintering, or metal 3D printing, the flow performance of powders directly determines the uniformity and performance of the final product.
How is powder flowability effectively to be enhanced then? Nanoscale oxide powders made through vapor deposition—magnesium oxide (MgO), aluminum oxide (Al₂O₃), and silicon dioxide (SiO₂)—are found to be important flow aids owing to their unique physicochemical properties. What are these three components? How do they function in different application scenarios?
Nanoscale oxides enhance the flow of powders through three prevalent mechanisms:
The tiny oxide particles coat large powder particles, creating a barrier to prevent them from adhering to each other. Think of sprinkling flour over dough to prevent it from sticking to surfaces. Experiments confirm this effect in plain sight. 1% addition of nano-SiO₂ to medicine powders improves their flowability, reducing their resting angle from 45° to 35°.
We can modify oxide surfaces chemically to alter the way they interact with water. For powders used in pharmaceuticals that clump when wet, we employ hydrophobic SiO₂ treated specially to repel water. This renders powders free-flowing even under humid conditions.
Mixing different sizes of oxide particles allows powders to be packed closer together. Mixing 30nm and 5nm Al₂O₃ particles, for instance, creates a more compact packing structure that improves flow by 15% compared to the use of one particle size.
An understanding of these principles helps manufacturers select the most appropriate flow aid for their specific powder and process conditions.
Vapor deposition is a method for preparing ultrafine powders by decomposing volatile metal compounds or reacting them with other gases. Powders produced by this method typically exhibit high purity, nanoscale particle size, and controllable morphology.
Magnesium oxide powder is normally prepared by chemical vapor deposition (CVD). Magnesium vapor is pyrolyzed with oxygen at elevated temperature in a reactor to form MgO particles of nanoscale. Temperature and gas flow rates need to be controlled carefully to prevent any size variation of particles.
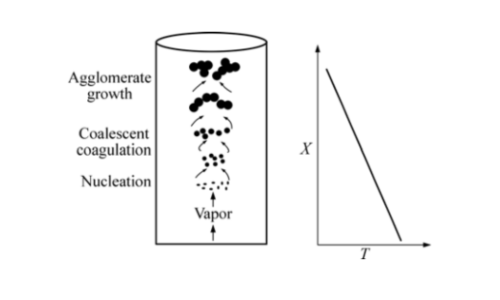
Fig.1 Schematic illustration of the processes of particle growth and gradient of reaction temperature in CVD.
Reaction Equation: 2Mg(g) + O₂(g) → 2MgO(s)
The resulting MgO powder is cubic in crystal structure with particle diameters of 20-100 nm and a specific surface area of 50-150 m²/g. It possesses a high melting point, making it suited for high-temperature applications.
Aluminum oxide powder is normally prepared by plasma-enhanced chemical vapor deposition (PECVD). Precursors are decomposed by plasma excitation to form nanoparticles at relatively low temperatures.
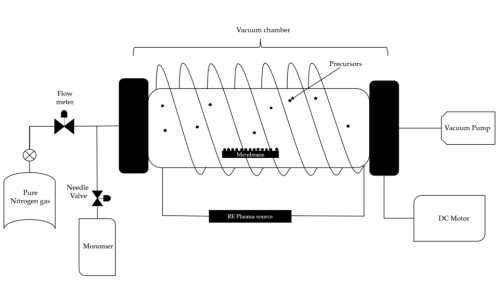
Fig 2. Plasma-Enhanced Chemical Vapor Deposition (PECVD)[1]
Reaction Equation: 4Al(g) + 3O₂(g) → 2Al₂O₃(s)
The obtained Al₂O₃ powder is composed of two common crystalline phases: high-temperature stable α-phase and high-surface-area γ-phase. The product contains smaller particle sizes (10-50 nm) compared to MgO and a specific surface area of up to 100-300 m²/g. It also has great thermal stability (>2000°C) and high chemical inertness, making it suitable for use in aggressive environments.
Silicon dioxide powder is typically prepared through flame hydrolysis, where silicon precursors are hydrolyzed at high temperatures in a hydrogen-oxygen flame to yield amorphous nanoparticles.
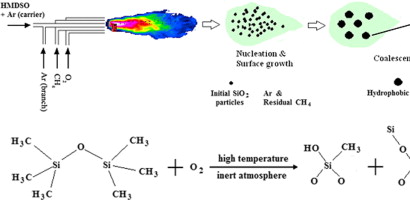
Fig 3. Hydrophobic SiO2 nanopaticles were fabricated by flame method with a branch of inert dilution gas[2]
Reaction Equation: SiCl₄(g) + 2H₂O(g) → SiO₂(s) + 4HCl(g)
The SiO₂ powder possesses a chain-type agglomerate structure with diameters of the particles ranging from 7-40 nm and a very high specific surface area of up to 400 m²/g. While its rich surface silanol groups (Si-OH) make easy chemical modification possible, they may also promote hydrogen bonding between particles.
The differences in preparation processes lead to significant variations in the physicochemical properties of these three materials, thereby influencing their performance as flow additives.
The three oxides each have their own strengths and exhibit distinct performance differences in practical applications.
MgO is suitable for high-density powders because of its high density, which prevents it from being blown away by air. Adding 0.5% nano-MgO to titanium alloy 3D printing improves powder flowability by 30%, significantly enhancing layer flatness. However, its hygroscopicity requires strict humidity control (RH <30%), increasing costs.
Al₂O₃ is highly chemical stable and performs very well in high-temperature injection molding of ceramic products. Addition of 1.5% γ-Al₂O₃ reduces the extrusion pressure of zirconia feedstocks by 25% without affecting sintered product density.
SiO₂ is most commonly used flow additive, particularly for low-density powders such as pharmaceuticals and foods. Hydrophobic SiO₂ is most used in the pharmaceutical sector to improve powder flowability.
|
Property |
MgO |
Al₂O₃ |
SiO₂ |
|
Optimal Temp. Range |
>800°C |
>1200°C |
<600°C |
|
Hygroscopicity |
High |
Low |
Adjustable |
|
Cost |
High |
Moderate |
Low |
|
Typical Dosage |
0.3–1% |
0.5–2% |
0.1–0.5% |
The three vapor-deposited oxide powders each have unique advantages as flow additives: MgO is suitable for high-temperature metal systems, Al₂O₃ excels in harsh environments, and SiO₂ dominates in conventional fields such as pharmaceuticals.
Stanford Advanced Materials (SAM) offers nano-scale magnesium oxide (MgO), aluminum oxide (Al₂O₃), and silicon oxide (SiO₂) powders. Particle sizes are customizable, and samples are available upon request.
[1] Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. https://doi.org/10.3390/nano13182586
[2] Renliang Yue, Dong Meng, Yong Ni, Yi Jia, Gang Liu, One-step flame synthesis of hydrophobic silica nanoparticles, Powder Technology, Volume 235, 2013, Pages 909-913, ISSN 0032-5910, https://doi.org/10.1016/j.powtec.2012.10.021.

United States
.png)



