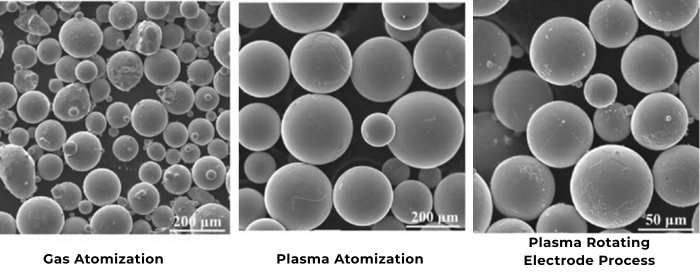
Silica and silicon powders are integral materials in various industrial, technological, and health-related fields. Despite their similar names, these substances have distinct characteristics and applications. This article delves into the properties, differences, and uses of silica and silicon powders, providing a comprehensive guide to understanding these versatile materials.

Silica, chemically known as silicon dioxide (SiO2), is a naturally occurring compound found in sand, quartz, and many other minerals. It is the most abundant mineral on Earth's surface, presenting a wide range of physical and chemical properties that make it invaluable across numerous industries.
Properties:
- Chemical Stability: Silica is highly resistant to chemical reactions, making it an excellent material for use in environments exposed to harsh chemicals.
- Thermal Stability: It can withstand high temperatures without decomposing, which is crucial for its use in manufacturing and construction.
- Hardness and Abrasiveness: Silica's hardness makes it suitable for applications requiring durable and abrasive materials.
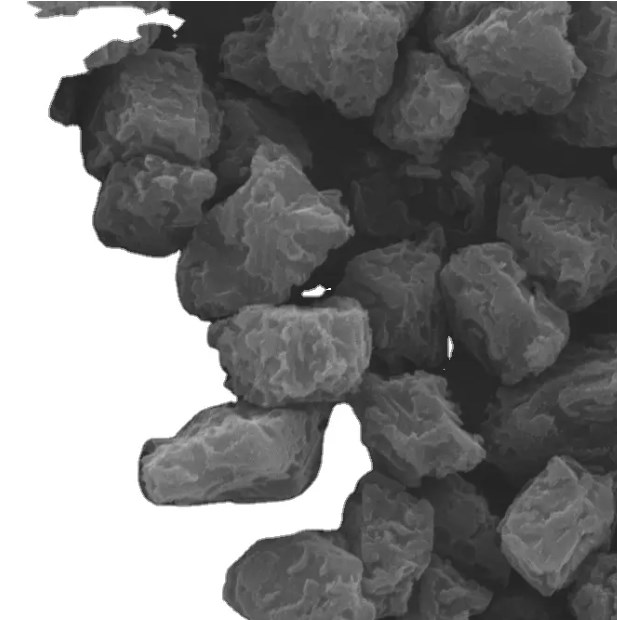
Uses:
- Construction Materials: Silica is a primary component in the production of glass, ceramics, and cement.
- Electronics: High-purity silica is used to make semiconductor chips and optical fibers due to its excellent insulating properties.
- Health and Beauty Products: Silica powder is commonly found in toothpaste, skin care products, and food supplements, where it acts as a thickening agent and provides essential nutrients.
Exploring Silicon Powder
Silicon, represented chemically as Si, is a metalloid element extracted from silica. It undergoes extensive processing to be transformed into a highly pure, powdered form. Silicon powder plays a crucial role in electronics and solar energy industries due to its semiconductor properties.
Properties:
- Semiconductor: Silicon's ability to conduct electricity under certain conditions makes it the backbone of modern electronics.
- Heat Resistance: Like silica, silicon can withstand high temperatures, which is vital for its use in electronic devices that generate heat.
- Photoconductivity: Silicon's capacity to convert light into electricity is exploited in photovoltaic cells for solar panels.
Uses:
- Electronics: Silicon is the primary material used in manufacturing microchips and transistors found in all electronic devices.
- Solar Panels: Silicon powder is used to produce solar cells that capture solar energy and convert it into electrical power.
- Alloys and Powders: Silicon powders are used in aluminum-silicon alloys to enhance their strength and castability, crucial for automotive and aerospace components.
What is the difference between silicone and silica and silicon?
While both materials originate from the same element, silicon, their applications and properties diverge significantly due to their different chemical compositions. Silica's wide availability and chemical inertness make it ideal for construction and manufacturing, whereas silicon's semiconductor properties are indispensable in electronics and renewable energy.
The terms silicone, silica, and silicon refer to distinctly different substances, each with unique properties and applications. Understanding the difference between them is crucial in fields ranging from industrial manufacturing to everyday consumer products.
Silicon
- Chemical Element: Silicon is a chemical element with the symbol Si and atomic number 14. It is a metalloid, meaning it has properties of both metals and non-metals.
- Natural Occurrence: It is the second most abundant element in the Earth's crust, primarily found as compounds like silica and silicates.
- Uses: Silicon is widely used in the electronics industry as a semiconductor material for integrated circuits and solar cells. It also serves as a base material in the production of silicones.
Silica
- Chemical Compound: Silica, or silicon dioxide (SiO2), is a natural compound made of silicon and oxygen. It's commonly found in quartz, sand, and many other minerals.
- Properties: Silica is known for its hardness, chemical inertness, and high melting point.
- Uses: It is used in various industries, including glass making, construction (concrete and mortar), ceramics, and as a filler in plastics and rubber. In its crystalline form, silica is used in hydraulic fracturing and filtration.
Silicone
- Polymers: Silicone refers to a group of synthetic polymers made up of silicon, oxygen, carbon, and hydrogen. Silicones can be liquids, gels, or solids.
- Properties: Silicones are known for their thermal stability, water repellency, flexibility, and low toxicity. They also exhibit resistance to UV light, ozone, and oxygen.
- Uses: Due to their versatile properties, silicones find applications in a wide range of products, including sealants, adhesives, lubricants, medical devices, cookware, and personal care products like shampoos and conditioners.
Key Differences
- Composition: Silicon is an elemental metalloid; silica is a compound of silicon and oxygen; silicones are polymers that include silicon among other elements.
- Physical State: Silicon is a solid crystalline substance; silica appears as crystalline quartz or amorphous sand; silicones can be engineered into various forms, including fluids, gels, and elastomers.
- Applications: Silicon is essential in electronics and solar cells; silica is used in glassmaking, construction, and filtration; silicones are utilized for their flexible, durable, and water-resistant properties in a myriad of consumer and industrial products.
Conclusion
Silica and silicon powders are foundational to the advancement of technology, construction, and even health products. Understanding their distinct properties and applications is essential for leveraging their benefits across various industries. As research and technology evolve, the potential uses for these versatile materials will continue to expand, further embedding them into the fabric of modern society.


![]()