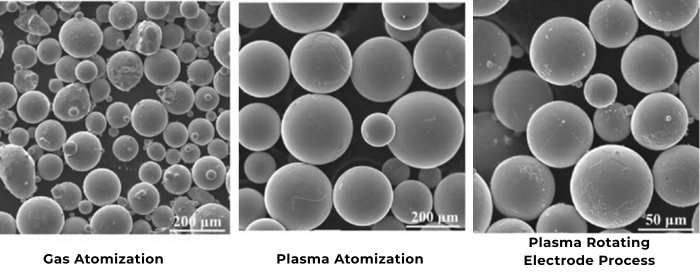
In the diverse world of material science, the measurement of particle size plays a pivotal role. The shape and size of particles can significantly impact the properties and behavior of materials, influencing their applications across various industries. Particle size analyzers have become indispensable tools in accurately characterizing these particles. However, the challenge often lies in dealing with the complexities presented by spherical and irregular-shaped particles.
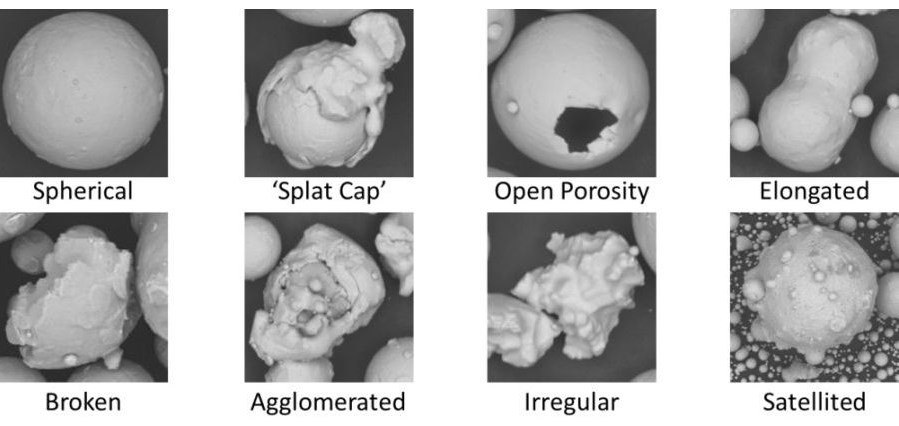
Particles come in an array of shapes - from perfectly spherical, like some types of pollen or certain manufactured beads, to highly irregular, such as crushed minerals or some types of synthetic powders. The shape of a particle can influence its flow properties, packing density, surface area, and reactivity.
These are often idealized in models due to their uniformity and symmetry. They tend to pack predictably and have a lower surface area compared to irregular particles of the same volume.
Spherical particles are often observed to flow better than their irregular-shaped counterparts. This improved flowability can be attributed to several key factors related to their shape and physical interactions:
Reduced Friction: Spherical particles have a lower surface area in contact with adjacent particles and surfaces compared to irregular particles. This reduced contact area minimizes friction, allowing them to roll and move past each other more easily. In contrast, irregular particles with rough or jagged surfaces create more friction and can interlock, which impedes flow.
Uniformity in Size and Shape: Spherical particles are more uniform in size and shape. This uniformity leads to fewer entanglements and less bridging (where particles get stuck in openings or between each other), contributing to smoother flow. Irregular particles, due to their varied shapes and sizes, are more prone to forming clumps and blockages.
Lower Angle of Repose: The angle of repose, which is the steepest angle at which a pile of unconsolidated particles remains stable, is generally lower for spherical particles. A lower angle of repose indicates better flowability, as the particles are less likely to form stable, non-moving heaps.
Better Packing: Spherical particles can pack more uniformly and often more densely, depending on the arrangement. This packing characteristic allows for smoother movement of particles as a collective mass, whereas irregular particles might have voids and inconsistent packing, leading to uneven flow.
Less Cohesive Forces: Due to their reduced surface area, spherical particles experience weaker cohesive forces like van der Waals forces. Less cohesion among particles further facilitates ease of movement, whereas irregular particles with larger surface areas might adhere to each other more strongly.
Predictable Behavior: The behavior of spherical particles under various conditions (like vibration, pressure, or gravitational forces) is more predictable and consistent. This predictability makes it easier to design and optimize systems (like hoppers, conveyors, and chutes) for handling and processing these particles.
These particles exhibit a multitude of shapes and sizes, making them more challenging to characterize. They can have higher surface areas and more complex packing behavior, which can affect their reactivity and mechanical properties.
Particle size analyzers utilize various techniques to measure particle size, each with its strengths and challenges when dealing with spherical and irregular particles.
This method, ideal for a broad size range, assumes particles are spherical to calculate size distribution. This assumption can lead to inaccuracies when analyzing irregular particles.
Best for: Wide range of particle sizes (from nanometers to millimeters).
Efficiency: Offers rapid and reproducible results.
Suitability: Ideal for both dry and wet samples.
Limitations: Assumes particles are spherical, which can affect accuracy for irregularly shaped particles.
Best suited for small particles, DLS can struggle with polydisperse samples or those with a mix of spherical and irregular shapes.
Best for: Very small particles, typically in the nanometer range.
Efficiency: Quick and suitable for small sample volumes.
Suitability: Used for particles in suspension.
Limitations: Less effective for polydisperse samples (samples with a wide range of particle sizes).
This technique provides direct visual information about particle shape and size. While it offers detailed insights, it can be time-consuming and may require a significant amount of data processing.
Best for: When shape characterization is as important as size.
Efficiency: Provides detailed information on particle morphology.
Suitability: Can be used for both dry and wet samples.
Limitations: Time-consuming and may require significant data processing.
A traditional method that is more suited for larger, irregular particles, but it lacks the precision of more modern techniques for fine particle sizes.
Best for: Larger particles, typically over 40 micrometers.
Efficiency: Simple and cost-effective.
Suitability: Ideal for dry, granular materials.
Limitations: Lower precision and cannot detect particles smaller than the mesh size of the sieves.
While offering high-resolution images and size measurements, electron microscopy is a more complex and costly technique.
Best for: Wide range of particle sizes.
Efficiency: Good for detailed size distribution analysis.
Suitability: Suitable for fine particles in suspension.
Limitations: Can be time-consuming and influenced by particle density.
Best for: Particles typically between 0.4 micrometers and 1,200 micrometers.
Efficiency: Fast and provides a direct count of particles.
Suitability: Ideal for cells, and large organic particles in suspension.
Limitations: Requires a conductive liquid and can be sensitive to particle shape.
The key to successfully navigating the complexities presented by different particle shapes lies in selecting the appropriate measurement technique and understanding its limitations:
Combining Techniques: Often, using a combination of techniques provides a more comprehensive understanding of particle size and shape. For example, laser diffraction for size distribution is complemented by image analysis for shape characterization.
Sample Preparation: Proper sample preparation, such as dispersion or agglomeration prevention, is crucial, especially for irregular particles, to ensure accurate measurements.
Understanding Limitations: Each technique has inherent limitations depending on particle shape. Acknowledging these limitations is essential in interpreting data correctly.
The characterization of spherical and irregular particles is a nuanced and complex task, critical in fields ranging from pharmaceuticals to materials engineering. Particle-size analyzers, equipped with advanced technologies and techniques, offer valuable insights into the world of particles. Understanding the strengths and limitations of each analytical method is crucial in obtaining accurate and meaningful data. As technology advances, the precision and ease of particle size analysis will continue to improve, enabling deeper insights into material properties and behaviors.