High-entropy alloy powders are metallic alloys composed of multiple principal elements (typically five or more) in nearly equal atomic ratios. Their defining feature is their "high configurational entropy."
Compared to traditional alloys, high-entropy alloy powders exhibit unique advantages in mechanical properties, oxidation resistance, thermal stability, and corrosion resistance. These powders are not only used to produce high-performance materials but also find extensive applications in extreme environments such as aerospace, nuclear industries, chemical equipment, and deep-sea engineering.
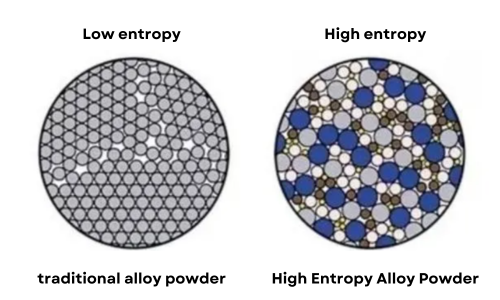
Fig 1. High Entropy Alloy Powder vs. Traditional Alloy Powder
|
Alloy Type |
Element Composition |
Characteristics |
Applications |
Particle Size |
|
Fe, Co, Ni, Cr, Ti |
High strength, corrosion resistance, thermal stability |
Aerospace, nuclear industries |
15–45 μm |
|
|
CoCrFeMnNi |
Co, Cr, Fe, Mn, Ni |
Excellent low-temperature toughness, pitting resistance |
Deep-sea engineering, cryogenic containers |
|
|
Hf, Nb, Ta, Ti, Zr |
High-temperature resistance, excellent oxidation resistance |
High-temperature structural components, engine parts |
Custom sizes available |
|
|
Ti, Zr, Mo, Hf, Ta |
High strength, wear resistance, corrosion resistance |
Nuclear reactors, chemical equipment |
Custom sizes available |
|
|
Ti, V, Nb, Mo, Ta, W |
Extremely high hardness, high-temperature corrosion resistance |
Aerospace engines, high-temperature furnaces |
Custom sizes available |
|
|
Co, Cr, Fe, Ni, Mo |
Excellent acid corrosion resistance, low porosity |
Chemical equipment, marine engineering |
15–53 μm or customized |
|
|
Al, Cu, Cr, Fe, Ni |
Wear resistance, high hardness, oxidation resistance |
Industrial cutting tools, automotive components |
Various sizes available |
In highly acidic environments, components made from CoCrFeNiMo alloy powder exhibit exceptional corrosion resistance. For example, in a 10% phosphoric acid solution, the corrosion current density is only 0.5 μA/cm², significantly lower than the 2.8 μA/cm² of 316 stainless steel. Additionally, its pitting potential reaches 0.2 V, more than twice that of stainless steel, reducing the risk of localized corrosion in acidic environments.
In high-temperature environments, HfNbTaTiZr alloys demonstrate excellent oxidation resistance. After 100 hours of oxidation testing at 1200°C, the oxidation layer thickness is only 10 μm, compared to 25 μm for traditional nickel-based alloys. Furthermore, its tensile strength at 1100°C is 350 MPa, 15% higher than that of nickel-based alloys, making it suitable for high-temperature structural components and aerospace nozzles.
The preparation method is a key factor influencing the properties of high-entropy alloy powders. The main techniques include:
|
Mechanical Alloying (MA) |
Raw powder materials are uniformly mixed using high-energy ball milling |
|
Gas Atomization (GA) |
High-temperature molten metal is atomized into spherical powders using high-speed gas flow |
|
Plasma Rotating Electrode Process (PREP) |
High-energy plasma melts rotating rods to produce high-purity powders. |
The preparation method directly determines the powder morphology, oxygen content, and microstructure, thereby affecting corrosion resistance.
|
Mechanical Alloying (MA) |
While simple, this method can introduce microcracks and impurities, potentially reducing passivation film performance. |
|
Gas Atomization (GA) |
Produces spherical powder particles with low oxygen content, significantly improving corrosion resistance. |
|
Plasma Rotating Electrode Process (PREP) |
Yields powders with extremely high purity and narrow particle size distribution, facilitating the formation of uniform passivation films. |
Read more:
Unleashing the Potential of High Entropy Alloy Powders: Preparation and Applications
Comparative Analysis of Different Preparation Methods of Spherical Powder
The corrosion resistance of high-entropy alloy powder largely depends on the types and proportions of alloying elements. Different elements function differently within the material, and their synergistic effects determine the alloy’s performance in corrosive environments.
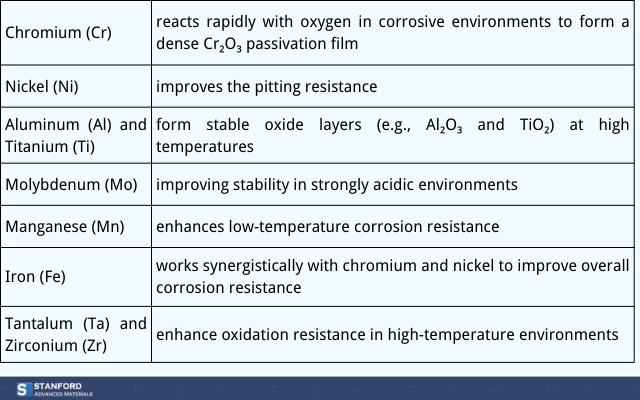
Chromium reacts rapidly with oxygen in corrosive environments to form a dense Cr₂O₃ passivation film, effectively preventing further corrosion. For example, increasing chromium content in AlCoCrFeNi alloys significantly reduces current density during electrochemical corrosion, enhancing pitting resistance.
Nickel significantly improves the pitting resistance of alloy powders, particularly in chloride-ion-rich environments. It stabilizes the passivation film and reduces localized corrosion. For example, CoCrFeMnNi components with high nickel content exhibit significantly lower corrosion rates in salt spray environments compared to standard stainless steel.
Aluminum and titanium form stable oxide layers (e.g., Al₂O₃ and TiO₂) at high temperatures, enhancing the high-temperature corrosion resistance of high-entropy alloys. For instance, the titanium content in HfNbTaTiZr powders provides excellent oxidation resistance in high-temperature tests.
Molybdenum promotes passivation film regeneration, improving stability in strongly acidic environments. High-entropy alloys containing molybdenum (e.g., MoNbTaW) demonstrate extremely low corrosion rates in concentrated sulfuric or phosphoric acids. Additionally, molybdenum reduces the risk of crevice corrosion.
.png)
Fig 2. Polarization curves of CoCrFeNiMox high entropy alloy coatings in 3.5% NaCl solution[1]
Manganese enhances low-temperature corrosion resistance by reducing grain boundary corrosion and improving toughness and crack resistance. For example, CoCrFeMnNi alloy components exhibit high stability in deep-sea, high-pressure corrosion experiments.
Iron, as a structural element, works synergistically with chromium and nickel to improve overall corrosion resistance. For example, iron in FeCrNiTiAl alloy powder enhances matrix strength while maintaining uniform passivation.
Tantalum and zirconium significantly enhance oxidation resistance in high-temperature environments. They form stable oxides in corrosive media, protecting the matrix. For example, HfNbTaTiZr alloys show oxidation rates at 1200°C that are half those of traditional nickel-based alloys.
In Summary: By optimizing alloying elements and their proportions, high-entropy alloy powders can achieve tailored performance for specific corrosive environments. For example, high-chromium and high-nickel compositions are ideal for high-salinity conditions, while molybdenum is critical for strong acidic environments.
High-entropy alloy powders, with their unique composition design and microstructure, exhibit exceptional corrosion resistance potential. Preparation methods determine powder quality and corrosion resistance, while alloying element optimization provides direction for further performance improvements.
[1] LIU Qian, WANG Xinyang, HUANG Yanbin, XIE Lu, XU Quan, LI Linhu. Effect of Molybdenum Content on Microstructure and Corrosion Resistance of CoCrFeNiMo High Entropy Alloy. Chinese Journal of Materials Research[J], 2020, 34(11): 868-874 DOI:10.11901/1005.3093.2020.269