

Imagine turning polluted water into clean water using nothing but sunlight—no longer a scene from science fiction. A research team at The Ohio State University has achieved a breakthrough in water purification technology by weaving titanium dioxide and copper into a three-dimensional nanofiber mat. This groundbreaking material not only captures sunlight like a solar cell but also actively breaks down poisons such as pesticides and industrial waste, even generating a small amount of electricity in the process.
The main drawback of traditional water purifying methods is the energy consumption. Titanium dioxide is a photocatalytic material, but its efficiency is not quite high. The team's innovation is to use technology for electrospinning to "knit" copper atoms into the nanostructure of titanium dioxide such that the material will be able to absorb more visible light and elevate photocatalytic efficiency to record-breaking levels. The experiments show that this nanofiber mat is superior to conventional solar-powered water cleaning systems under natural sunlight and remains stable even after more than 20 reuse cycles.
But why does titanium dioxide and copper create such a great synergy?
Titanium dioxide is a conventional photocatalyst that exhibits structural stability and high surface activity. When exposed to ultraviolet (UV) light, TiO₂ generates extremely reactive free radicals (such as ·OH) which oxidize and degrade organic contaminants—such as pesticides, dyes, and industrial waste effluents—into nontoxic carbon dioxide and water.
Fig 1. TiO2‐based artificial photosynthesis[1]
Nano-titanium dioxide possesses unique characteristics, including high surface area, strong magnetic response, strong light absorption, strong surface reactivity, good thermal conductivity, and good dispersibility. Nano-TiO₂ also possesses stable photochemical activity, strong catalytic activity, strong oxidative ability, is non-toxic, cost-effective, easy to process, and does not result in secondary pollution. Thus, nano-titanium dioxide has been studied and applied extensively in environmental processes like wastewater treatment and air purification.
Note: NOx (oxides of nitrogen) are major pollutants responsible for causing the greenhouse effect, acid rain, depletion of the ozone layer, and photochemical smog.
Traditional titanium dioxide has a fatal disadvantage: it can utilize merely 5% of sunlight (UV spectrum), resulting in low catalytic activity and precluding large-scale practical applications. However, the hybridization of TiO₂ with copper shatters this barrier.
Copper is added, which extends titanium dioxide light absorption from UV to visible spectra (45% of sunlight), significantly improving solar energy harvesting. Copper also forms a heterojunction with TiO₂, which accelerates the separation of electron-hole pairs and thereby enhances free radical generation.
Interestingly, copper itself also assists in degrading the pollutants. Copper, when in contact with oxygen and water, through chemical reactions, produces hydroxyl radicals (·OH) and reactive oxygen ions (O₂⁻), which enable adsorption and degradation of specific contaminants through redox reactions.
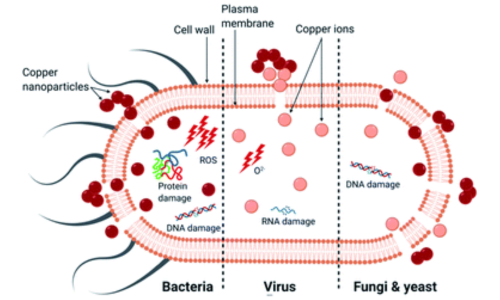
Fig 2. The primary mechanism of death in different microorganisms by copper nanoparticles[2]
The synergistic combination of titanium dioxide and copper creates new possibilities for photocatalyst-based water purification. While TiO₂'s stable photochemistry and high catalytic efficiency make it an ideal material for environmental remediation, its requirement for UV light has traditionally limited practical applications. Not only does the addition of copper broaden its light-responsive spectrum, but it also enhances the efficiency of electron transfer, enabling this nanocomposite to realize superior purification even under visible light.
As a professional and high-quality material supplier, Stanford Advanced Materials (SAM) provides premium raw materials—such as the titanium dioxide and copper covered in this report—to support frontier research and new applications. Academic and research institutes can receive SAM's special discounts.
[1] Ruan, Xiaowen & Li, Shijie & Huang, Chengxiang & Zheng, Weitao & Cui, Xiaoqiang & Ravi, Sai. (2023). Catalyzing Artificial Photosynthesis with TiO2 Heterostructures and Hybrids: Emerging Trends in a Classical yet Contemporary Photocatalyst. Advanced Materials. 36. 10.1002/adma.202305285.
[2] Salah, Intisar & Parkin, Ivan & Allan, Elaine. (2021). Copper as an antimicrobial agent: Recent advances. RSC Advances. 11. 18179-18186. 10.1039/D1RA02149D.

United States
.png)



