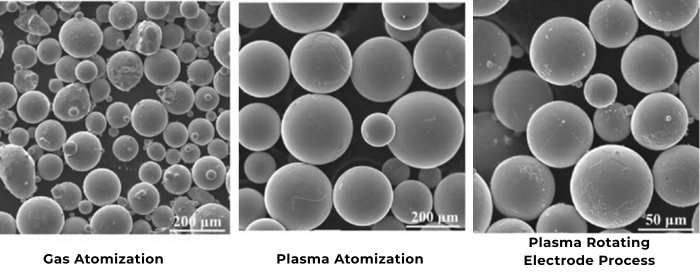
Silver powder (Ag powder) is primarily categorized by morphology into spherical silver powder, flake silver powder, dendritic silver powder, etc. Morphology exerts a great influence on its performance and applications. The electronics industry is the primary application field for Ag powder, accounting for approximately 60%-70% of the world's silver particles consumption. As an example, we will illustrate the impact of morphology on its properties and applications in the electronics industry.
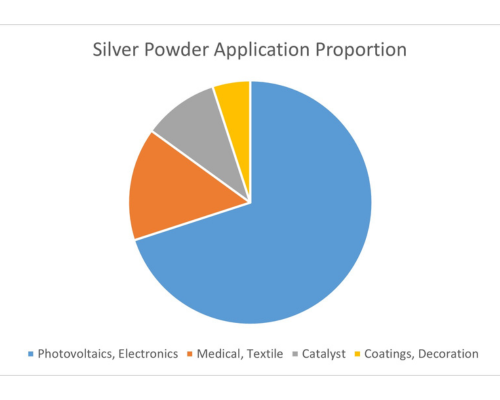
Fig 1. main applications of Ag powder
As shown in Figure 1, the main applications of silver powder include photovoltaics, electronics, medical and textiles, catalysts, and coatings. Among these, growing demand for photovoltaic cells and electronic products is the most important driver for the Ag powder market.
The rapid development of the electronics sector has driven the application of conductive pastes, a very simple base material. Conductive pastes are applied by integrated circuits, chip components, microwave devices, solar cells, and other microelectronic components. Conductive silver paste, a very significant auxiliary material in the manufacture of solar cells, plays a significant role in the photoelectric conversion efficiency of solar cells, particularly in the solar energy industry.
Powdered silver is the principal raw material employed for producing silver paste that serves as the conductive component and plays a crucial role in determining the cell performance. Its morphology determines solar cell performance directly or indirectly.
Why Does Silver Powder Morphology Affect Cell Performance?
This is because particles that have complex shapes on their surface have higher surface free energy, thereby agglomeration. This leads to sintering deformation and shrinkage of the silver paste and affects its performance. However, on the other hand, increased surface roughness and microporosity of the Ag powder also increase the adhesion between the solar cell and the silver paste.
Different surface topography of Ag powder are compatible with different uses.

Fig 2. Microscopic images of Ag powder with different appearances
Spherical silver powder has a high sphericity, thus, the silver paste is very fluid when formulated. The paste may then flow freely through fine grid lines, making spherical silver powder highly compatible with front-side silver paste for solar cells. Spherical Ag powder of micron or submicron size is already being utilized on a large scale in solar cell front-side pastes.
Compared to back-side paste, front-side paste needs higher conductivity. To achieve a sufficient contact area, producers will combine large and small particle sizes.
Flake silver powder has wide applications in microelectronics, flexible displays, photovoltaics, and so on.
Made by processing spherical Ag powder, silver flake powder has a specific two-dimensional structure that provides increased contact area in silver paste compared to other morphologies. This subsequently results in the reduction of resistance and increased conductivity. Sintering density is also increased with flake structure.
Due to its extensive surface area, flake silver paste can deliver greater coating coverage for the same weight, reducing the silver content and coating thickness while maintaining the conductivity. Silver flake also offers greater flexibility and crack resistance for improving the reliability of electronic devices.
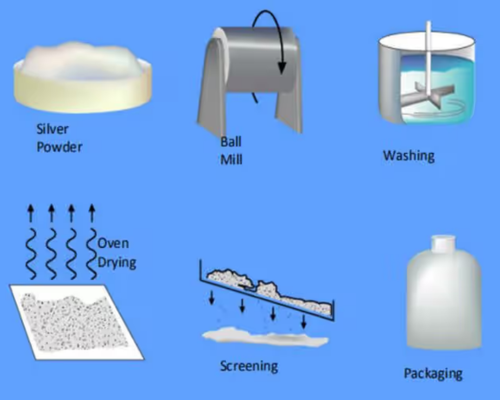
Figure 3. Production Process of Silver Flake
However, flake silver paste is not as fluid, which renders it unsuitable for high-precision front-side solar cells' electrodes. Rather, it is used in back-side uses, where accuracy can be poor, basically saving money while still maintaining low resistivity.
Silver dendritic powder consists of particles of silver organized in a branched framework having high surface energy and agglomeration tendency. This leads to porous and low-conductivity thick films upon sintering, making it unsuitable for solar cell pastes.
But in conductive adhesives, its irregular shape is useful. The dendritic shape has greater particle contact probability and forms a conducting network at a mere 40% filler loading, with low resistivity being obtained. Silver powder in the flaky shape, although with low resistivity due to larger contact area, requires 70% filler loading for maximum conductivity due to low dispersibility.
The morphology of silver powder significantly affects its conductivity, fluidity, sintering density, and other properties.
Table 1. Comparison of Different Ag Powder Morphologies
|
Property |
Spherical Silver Powder |
Flake Silver Powder |
Dendritic Silver Powder |
|
Morphology |
Smooth spherical particles |
Flat flakes with high aspect ratio |
Branched structure, prone to agglomeration |
|
Conductivity |
High |
Moderate to high |
Moderate |
|
Fluidity |
Excellent |
Poor, tends to stack |
Poor, agglomerates easily |
|
Sintering Density |
High |
Very high |
Low, porous structure |
|
Suitable Processes |
High-temperature sintering, 3D printing |
Low-temperature curing, screen printing |
Conductive adhesives |
|
Main Applications |
Front-side PV paste, electronic packaging |
Back-side PV paste, flexible circuits, RFID |
Conductive adhesives, EMI shielding |
|
Typical Uses |
Solar cell grid lines, chip bonding |
Touchscreen circuits, wearable devices |
Electronic bonding, anti-static coatings |
In practical applications, the choice of silver powder is not limited to a single morphology. Hybrid systems and morphology optimization can further enhance performance and reduce costs. For example, back-side PV paste often uses a mix of spherical and silver flake. Beyond morphology, particle size must also be considered. Generally, micron-sized silver powder offers a balance of performance and cost efficiency.
Stanford Advanced Materials (SAM) offers silver powders in various morphologies and particle sizes. If you are interested, please click the link below to view detailed product information: Silver. Alternatively, you can contact our professional sales team through Get A Quote.