

Austenitic stainless steel is one of the most common types of stainless steel, accounting for approximately 70% of global stainless steel production. It possesses sufficient strength, excellent plasticity, and relatively low hardness, which are among the reasons for its widespread adoption. This type of stainless steel contains a high proportion of nickel and chromium, and these two elements have distinct effects on its properties.
Nickel-chromium steel typically refers to austenitic steel containing nickel and chromium, especially common types such as 304 and 316 SS. Given their high nickel and chromium content, these steels form a stable austenitic structure, exhibiting excellent corrosion resistance and mechanical properties. Therefore, it can be said that most nickel-chromium stainless steels belong to the austenitic category.
However, not all stainless steels containing nickel and chromium have an austenitic structure. Some martensitic steels also contain nickel and chromium. Their main characteristic is the presence of a martensitic phase, which provides high hardness and strength. Typical examples include models such as 410 and 420.

Fig 1. Nickel-Chromium Steel
Chromium is a fundamental element in stainless steel powder and the most critical element determining corrosion resistance. By definition, stainless steel must contain a minimum of 10.5% chromium. The higher the chromium content, the better the corrosion resistance. This is because chromium enables the formation of a stable surface protective film (approximately 10 μm) based on Cr₂O₃ in oxidizing environments, leading to passivation. This chromium-rich oxide film exhibits good stability in many media, thereby enhancing the corrosion resistance. Additionally, chromium effectively increases the electrode potential of austenite, shifting the potential of pure iron (low-carbon steel) from negative to positive, making the steel resistant to corrosion.
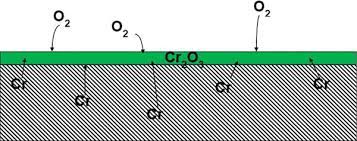
Fig 2. Chromium makes stainless steel stainless
The role of chromium in stainless steel is not limited to providing corrosion resistance. Through solid solution strengthening, chromium significantly enhances the hardness and strength of stainless steel. When chromium atoms dissolve into the iron lattice, the difference in atomic radii between chromium and iron causes lattice distortion. This distortion increases the dislocation density within the material, hindering dislocation movement and thereby enhancing the material's strength and hardness.
Furthermore, chromium positively influences the mechanical and processing properties of stainless steel powder. It slows down the transformation rate of austenite to ferrite and carbides and reduces the critical cooling rate for quenching.
It is worth noting that an increase in chromium content may affect the sintering dynamics of the powder. High chromium content typically requires higher sintering temperatures to achieve sufficient densification.
Nickel is a potent austenite stabilizer, maintaining the face-centered cubic (FCC) structure of the material. It effectively prevents the transformation of austenite into ferrite or martensite during cold working or heat treatment.
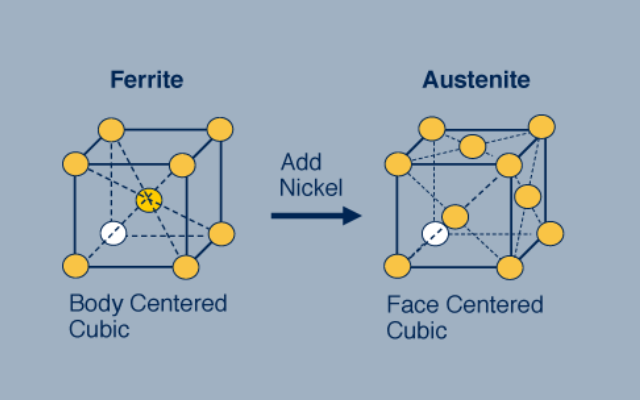
Fig 3. By adding nickel, the structure changes from bcc to face centered cubic (fcc)
Nickel is the second most important alloying element in stainless steel powder after chromium. To resist corrosion from acids and alkalis, chromium alone is insufficient, and nickel must be added. Nickel promotes the stability of the passive film on stainless steel and enhances its thermodynamic stability. Therefore, the coexistence of chromium and nickel in stainless steel significantly strengthens its rust resistance and corrosion resistance. High nickel content imparts better ductility and plasticity to austenitic stainless steel powder, making it easier to deform and densify during sintering, thereby reducing sintering defects. At the same time, it improves the material's overall toughness, enhancing its resistance to impact and fracture.
In powder form, nickel has a certain influence on processability. Higher nickel content generally helps lower sintering temperatures, increase sintering rates, and promote powder densification. However, the presence of nickel can also affect powder flowability. High nickel content may increase inter-particle attraction, impacting flow performance, which requires optimization of powder morphology and size distribution to address.
The rational regulation of nickel and chromium is crucial for optimizing the comprehensive properties of austenitic stainless steel powder. Chromium enhances corrosion resistance, hardness, strength, wear resistance, and thermal stability, ensuring material reliability in harsh environments. Nickel, on the other hand, improves toughness, ductility, and corrosion resistance, enhancing material performance under complex stress conditions.
Typical Austenitic Stainless Steel Powder (e.g., 304, 316):
High-Nickel Austenitic Stainless Steel Powder (e.g., 310):
Stanford Advanced Materials (SAM) is at the forefront of powder development and offers a wide range of stainless steel powder types and grades. For more information on these powders please contact us and check out our pages.

United States
.png)



